The Aluminum Production Process—from Art To Science
Throughout
the years since its invention in 1886, the industrial aluminum
production has developed from art to science. Steadily increased
understanding of the process has been achieved as a result of extensive
research and development work, particularly in the latter half of the
twentieth century, both in aluminum plants and in several universities
and academic institutions. During monitoring and intervention of the
process, the cell operators are constantly faced with decision-making
situations. Theoretical and practical training of the operators and
their supervisors and superintendents give them the skills and knowledge
needed to improve steadily cell operation and work practices.
The overall electrochemical reaction for industrial production of molten aluminum may be written as follows:
This reaction is simple and shows that the two main raw materials are alumina and carbon and that there are two chemical products, molten aluminum, which we want, and gaseous CO2, which we really do not want.
The amounts of raw materials used in the process are illustrated in Fig. 2. Alumina is consumed according to the stoichiometric ratio predicted from Equation 1. The consumption of alumina theoretically amounts to 1.89 kg per kilogram of aluminum produced. Nevertheless, in practice, the real value for the specific alumina consumption in the industry is a little higher, typically 1.93 kg, because the alumina supplied is not 100% pure. It always contains small amounts of impurity oxides like Na2O, CaO, Fe2O3, and SiO2. Furthermore, from the aforementioned chemical equation, we see that we produce three-fourth moles of CO2 per mole of aluminum. One-half mole of alumina should then theoretically react with 0.33 kg of carbon and produce 1 kg of aluminum and 1.22 kg of CO2. Nevertheless, because of other reactions of carbon with both oxygen and CO2, between 0.40 and 0.45 kg carbon are consumed per kilogram of aluminum in practice. This is called the net anode consumption, and this in turn produces about 1.5 kg of CO2 per kilogram of aluminum.
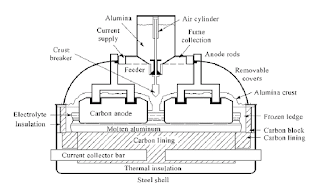
Alumina must be added regularly to the electrolyte to keep the normal electrolytic production going on continuously. Older aluminum electrolysis cell designs had large and infrequent additions of alumina, while modern cells are equipped with so-called point feeders. Alumina is then supplied automatically from an overhead bin or hopper, which is built into the superstructure of the cell. Two to six volumetric feeders successively add about 1 kg of alumina to the electrolyte every minute or so. These small additions increase the ability for the alumina powder to dissolve, mix, and disperse rapidly in the electrolyte. The average alumina concentration in the electrolyte is usually kept within the narrow range of 2 to 4 wt% alumina. Higher concentrations may lead to the formation of excessive amounts of undissolved alumina, which in the industry is called sludge. Because of its higher density, the sludge is collected at the bottom of the molten metal. Sludge has no useful purpose in the cell, and it is unwanted, mainly because it contributes to increase the electrical resistance in the cell and thereby the cell voltage.The amounts of raw materials used in the process are illustrated in Fig. 2. Alumina is consumed according to the stoichiometric ratio predicted from Equation 1. The consumption of alumina theoretically amounts to 1.89 kg per kilogram of aluminum produced. Nevertheless, in practice, the real value for the specific alumina consumption in the industry is a little higher, typically 1.93 kg, because the alumina supplied is not 100% pure. It always contains small amounts of impurity oxides like Na2O, CaO, Fe2O3, and SiO2. Furthermore, from the aforementioned chemical equation, we see that we produce three-fourth moles of CO2 per mole of aluminum. One-half mole of alumina should then theoretically react with 0.33 kg of carbon and produce 1 kg of aluminum and 1.22 kg of CO2. Nevertheless, because of other reactions of carbon with both oxygen and CO2, between 0.40 and 0.45 kg carbon are consumed per kilogram of aluminum in practice. This is called the net anode consumption, and this in turn produces about 1.5 kg of CO2 per kilogram of aluminum.
On the contrary, low alumina concentrations in the electrolyte can give a dramatic change in the anode process, which leads to a so-called anode effect. An anode effect causes a very high cell voltage, perhaps up to 30 to 40 V instead of the normal 4.0 to 4.5 V, by forming an electrically insulating layer of gas underneath the anodes. The anode gas composition then changes abruptly from almost pure CO2 (g) to mainly CO (g) and also some gaseous perfluorocarbon compounds, CF4 (g) and smaller amounts of C2F6 (g). These are greenhouse gases with high global-warming potential and extremely long atmospheric life times (of the order of 10,000 years).
The formation of these gases can be lowered by reducing the anode effect frequency (the number of anode effects per cell per day) and the anode effect duration (given in minutes). All aluminum producers have now made significant progress in reducing their emissions of perfluorocarbon gases. Most modern prebake cells can now be controlled to operate for more than 1 week and even for several months without an anode effect.
Before leaving the topic of anode effects, it should be mentioned that 70% to 80% of the anode gas evolved is then CO (g). In some cases, termination of anode effects may require manual intervention, and the operators may then breathe in this poisonous gas. Nevertheless, even if this effect has probably not been studied in detail, the concentration of CO (g) in the working atmosphere in potlines may be so low that it is not harmful to humans.
In addition to being the raw material for production of aluminum, alumina also acts as a thermal insulator when it is placed on top of the self-formed solid crust above the electrolyte, thereby reducing heat losses. Alumina is also used for covering the top of the anodes, which conserves heat and minimizes air burning of the carbon anodes. More frequently, a mixture of alumina powder and crushed pieces of solid electrolyte is used.
The third major role fulfilled by alumina is a very important one. Alumina is used to capture fluoride emissions from the cells by anode gas cleaning, by use of the so-called dry scrubbing method. Alumina powder adsorbs the hydrogen fluoride (HF) gas evolved, and it also entraps fluoride condensates, mainly particulate sodium tetrafluoroaluminate (NaAlF4). The resulting alumina is called secondary alumina and is then used as feed material to the cells. The cleaned exhaust gas, containing CO2 and smaller amounts of perfluorocarbon gases, is discharged to the atmosphere.
Figure 3 shows a flow sheet of the industrial aluminum production process. The processes made before the metal is sent to the cast house are called upstream processes, while the processes in the cast house to make extrusion ingots, sheet ingots, primary foundry alloys, and/or wire rods are called downstream processes. A much more detailed description of the electrolysis process can be found in several textbooks, for example, in Grjotheim and Kvande2 and Thonstad et al.3
Alumina must be added regularly to the electrolyte to keep the normal electrolytic production going on continuously. Older aluminum electrolysis cell designs had large and infrequent additions of alumina, while modern cells are equipped with so-called point feeders. Alumina is then supplied automatically from an overhead bin or hopper, which is built into the superstructure of the cell. Two to six volumetric feeders successively add about 1 kg of alumina to the electrolyte every minute or so. These small additions increase the ability for the alumina powder to dissolve, mix, and disperse rapidly in the electrolyte. The average alumina concentration in the electrolyte is usually kept within the narrow range of 2 to 4 wt% alumina. Higher concentrations may lead to the formation of excessive amounts of undissolved alumina, which in the industry is called sludge. Because of its higher density, the sludge is collected at the bottom of the molten metal. Sludge has no useful purpose in the cell, and it is unwanted, mainly because it contributes to increase the electrical resistance in the cell and thereby the cell voltage.
On the contrary, low alumina concentrations in the electrolyte can give a dramatic change in the anode process, which leads to a so-called anode effect. An anode effect causes a very high cell voltage, perhaps up to 30 to 40 V instead of the normal 4.0 to 4.5 V, by forming an electrically insulating layer of gas underneath the anodes. The anode gas composition then changes abruptly from almost pure CO2 (g) to mainly CO (g) and also some gaseous perfluorocarbon compounds, CF4 (g) and smaller amounts of C2F6 (g). These are greenhouse gases with high global-warming potential and extremely long atmospheric life times (of the order of 10,000 years).
The formation of these gases can be lowered by reducing the anode effect frequency (the number of anode effects per cell per day) and the anode effect duration (given in minutes). All aluminum producers have now made significant progress in reducing their emissions of perfluorocarbon gases. Most modern prebake cells can now be controlled to operate for more than 1 week and even for several months without an anode effect.
Before leaving the topic of anode effects, it should be mentioned that 70% to 80% of the anode gas evolved is then CO (g). In some cases, termination of anode effects may require manual intervention, and the operators may then breathe in this poisonous gas. Nevertheless, even if this effect has probably not been studied in detail, the concentration of CO (g) in the working atmosphere in potlines may be so low that it is not harmful to humans.
In addition to being the raw material for production of aluminum, alumina also acts as a thermal insulator when it is placed on top of the self-formed solid crust above the electrolyte, thereby reducing heat losses. Alumina is also used for covering the top of the anodes, which conserves heat and minimizes air burning of the carbon anodes. More frequently, a mixture of alumina powder and crushed pieces of solid electrolyte is used.
The third major role fulfilled by alumina is a very important one. Alumina is used to capture fluoride emissions from the cells by anode gas cleaning, by use of the so-called dry scrubbing method. Alumina powder adsorbs the hydrogen fluoride (HF) gas evolved, and it also entraps fluoride condensates, mainly particulate sodium tetrafluoroaluminate (NaAlF4). The resulting alumina is called secondary alumina and is then used as feed material to the cells. The cleaned exhaust gas, containing CO2 and smaller amounts of perfluorocarbon gases, is discharged to the atmosphere.
Figure 3 shows a flow sheet of the industrial aluminum production process. The processes made before the metal is sent to the cast house are called upstream processes, while the processes in the cast house to make extrusion ingots, sheet ingots, primary foundry alloys, and/or wire rods are called downstream processes. A much more detailed description of the electrolysis process can be found in several textbooks, for example, in Grjotheim and Kvande2 and Thonstad et al.3
Our company
Zhengzhou Joda Technology Co., Ltd. is a modernized comprehensive enterprise integrating research, manufacture, export and technical service of various types of equipments for aluminum smelters all over the world. Our products: automatic anode jacking frame, ladles accessories, aerogel insulation blanket, anode jacking system, Anode Clamp, bimetal, aluminum ladle cleaning machine, anode rod and MTV tapping tube cleaner etc.
aerogelinsulationblanket.com
saleaerogel.com
sellaerogel.com
anodejackingframe.com
aerogelinsulationblanket.com
saleaerogel.com
sellaerogel.com
anodejackingframe.com




评论
发表评论